An orchard in the distance may seem to be uniform greenish foliage, but when you drive by an orchard you witness first hand that there are certain angles at which you can see all the way through the trees. Even when driving through a desert one can detect patterns arising from the plant spacing to accommodate their demands for the limited supply of moisture in the soil. But nature (as against man-made constraints to accommodate mechanical access) tends to partition resources so as to equally divide surface area into hexagonal regions that minimize boundaries that separate segmented portions of the surface as in honeycomb.


The surface of a table seems smooth and uniform, but when viewed through an electron microscope it is a very different matter. The existence of hexagonal structure as in honeycomb becomes apparent when viewing solid surfaces under an electron microscope.
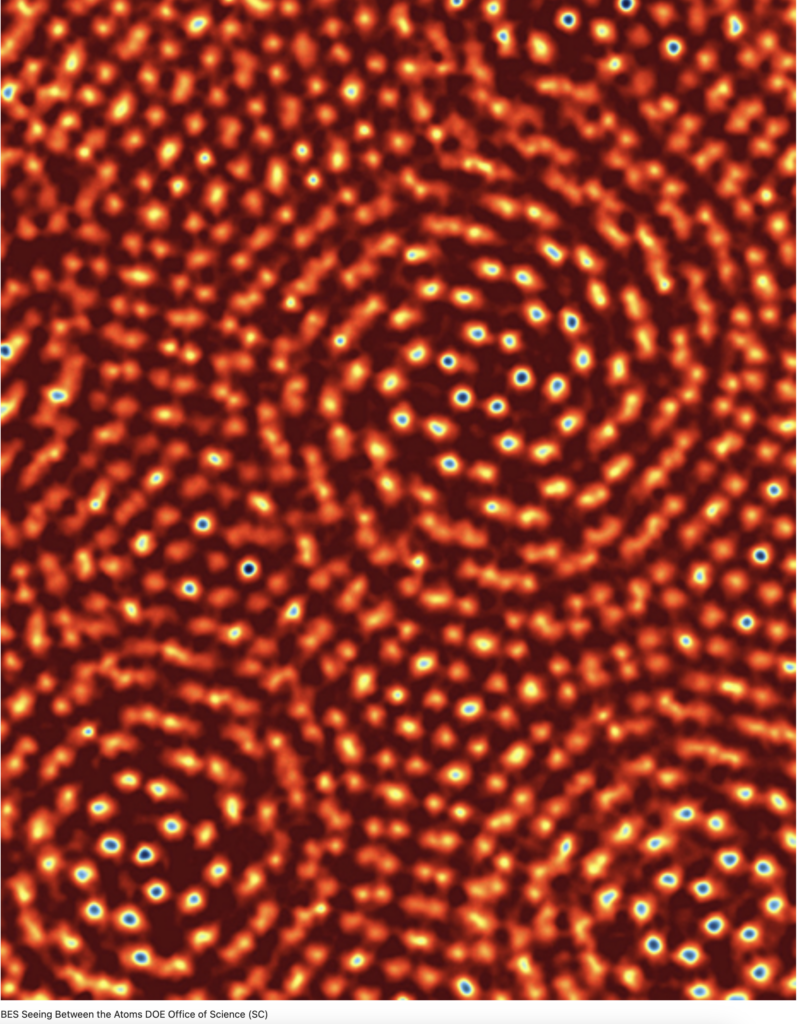
But the two-dimensional similarity stops there. The individual atoms or molecules of which the table or other solid is comprised share interfaces that for spherical objects are rhombic dodecahedrons, sharing 12 flat surface boundaries with 12 other objects butted up against the center of each planar surface.
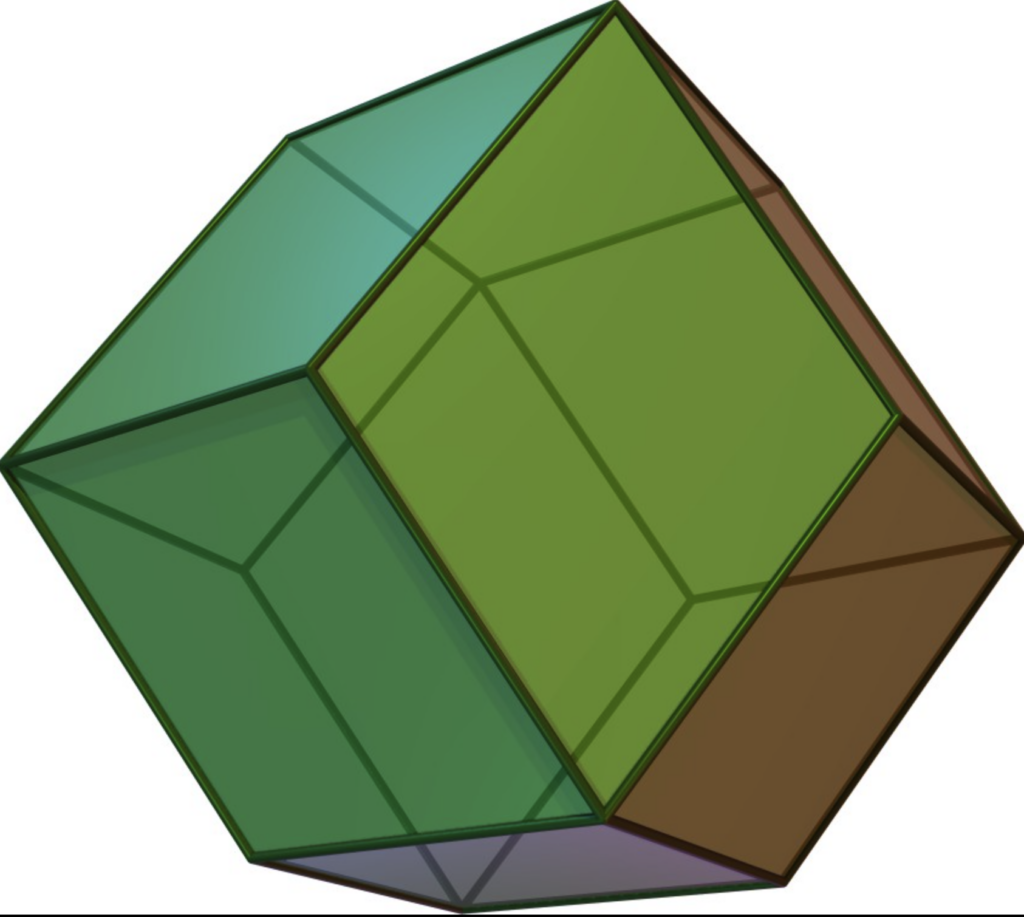
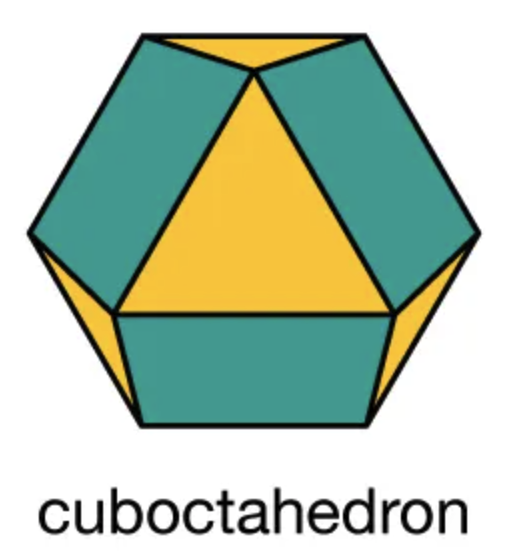
These twelve objects (one per face of the rhombic dodecahedron of a central object) are arranged such that their centers define the vertices of a cuboctahedron.
rhombic dodecahedron
When looking into such solids with an electron microscope there are angles at which one can see deeper into the substance analogous to looking through the the two dimensional rectilinear structure of an orchard. That is because the individual objects are but a part of a stucture made up of these objects and groups of objects. The entire volume of the solid is a single structure like a jiggled box of golf balls that will compact with hexagonal layers offset from one another to effect the minimal occupation of space. In the diagram below the occupancy of space is reduced from the orchardly stacks of egg cartons, to the honeycomb structure, to the most compact stacking of spherical objects.
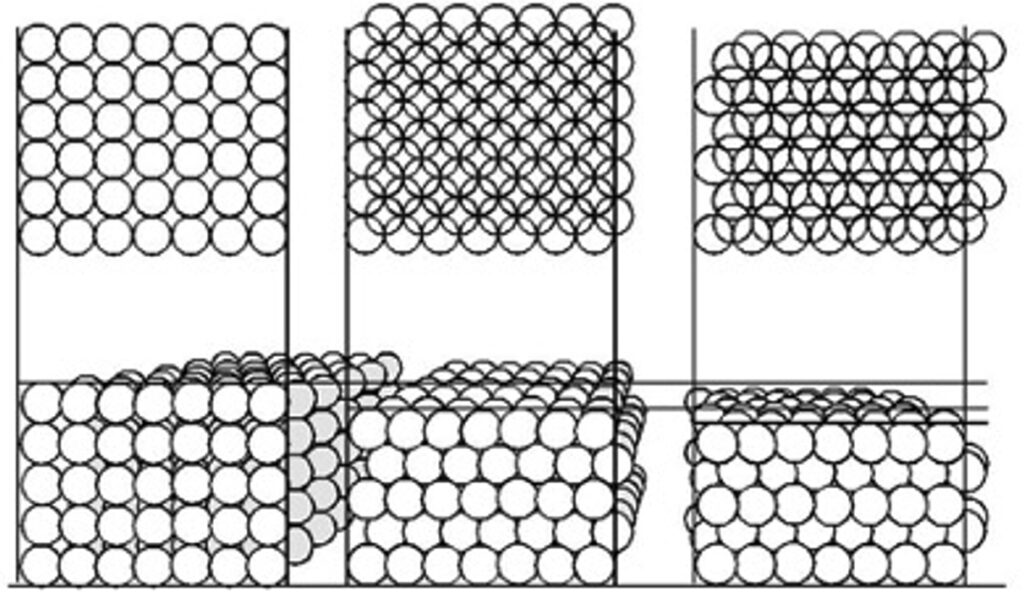
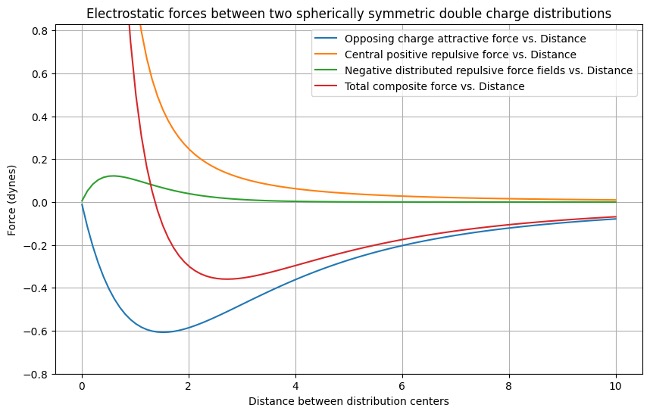
Forces of gravitational and thermodynamic pressure in galaxy clusters are analogous to Van der Waals forces between atoms and molecules producing the cuboctahedral structures that provide observation perspectives analogous to naked eye views through an almond orchard or by an electron microscope through the atoms or molecules in a solid. Most lines of sight will not be through adjacent objects in such structures as illustrated by cuboctohedral stacks of Tycho Brae’s cannon or my golf balls.
Leave a Reply